Oxygen: Friend or Foe?
/Shout out to Chelsea Jorgenson, nurse at UW Medical Center, for the episode idea!
Oxygen is a drug.
The FDA regulates medical gases like oxygen as a drug, with the approved indications of hypoxia and hypoxemia. Did you know these are different?
Hypoxemia: reduced partial pressure of O2 in the blood (low PaO2).
Hypoxia: reduced tissue levels of O2 so that cellular metabolism is impaired.
Hypoxemia generally precedes hypoxia.
You have less oxygen to deliver, so there’s less O2 in the tissues over time.
Hypoxemia does not always result in hypoxia.
For instance, those who live at high altitude can by hypoxemic, but not hypoxic.
Like other drugs, oxygen has benefits, but also has potential harms. And for a quick review of the benefits/harms of oxygen medically, check out the Journal of Hospital Medicine’s series, “Things We Do For No Reason.”
Many studies have shown, mostly on animal models, that hyperoxygenation leads to lung injury, inflammation through free radical generation, and changes in perfusion that may actually be harmful:
COPD: oxygen titrated to goal >88-92% is associated with 2x fold increased mortality risk.
Linked likely due to worsened ventilation-perfusion matching and poorer CO2 offloading as PaO2 rises (Haldane effect).
MI: 1976 RCT of O2 in suspected MI patients at 6L/min. Patients receiving for 24 hours or more had more episodes of tachycardia with no improvement in mortality, analgesic use, or infarct size.
Subsequent trials have found similar outcomes, and actually have also demonstrated increased rate of MI recurrence with O2 use.
European Society of Cardiology has now actually recommended no O2 use unless SpO2 < 90% for MI patients!
Retinopathy of prematurity: hyper oxygenation of neonates increases risk of blindness.
Other illness: trials in settings ranging from ICUs, strokes, TBIs, and cardiac arrest have also linked liberal O2 use (ranging from 2L NC upwards) to increased mortality and other adverse events.
A meta analysis demonstrated a dose-dependent toxicity: for every 1% increase of SpO2 above 94-96%, there was 25% relative increase in in hospital mortality!!!
So when is oxygen helpful?
Importantly, these studies have mostly looked at normoxemic patients who receive supplemental oxygen. Patients who are significantly hypoxemic or hypoxic will certainly benefit from O2.
Additionally, patients with conditions such as CO poisoning, cluster headaches, sickle cell crisis, and pneumothorax may all benefit from O2. These are actual indications for the drug.
OK, so what about pregnancy and labor?
O2 is most commonly administered in labor, in an attempt to improve fetal status. The thought being that, if we see significant decelerations that reflect fetal hypoxia, administration of supplemental oxygen through the mother/placenta will help to correct it.
Pro oxygen evidence:
Fetal pulse oximetry studies 2 small studies using a fetal pulse-oximeter in laboring women demonstrated increased fetal oxygenation of 5% with simple face masks, and 7-15% when using non-rebreathers, in non-hypoxic fetuses. In hypoxic fetuses, the observed benefit was greater, 20% with simple face mask and 26-37% with non-rebreather.
Fetal pulse oximetry did not help to improve rates of cesarean delivery for fetal indications, and thus has not caught on as a routine technology in labor management. Thus critics would argue it’s hard to interpret these studies in context of whether O2 improves neonatal outcomes, or just makes the saturation numbers better.
Anti-oxygen evidence:
Fetal scalp pH study:a small study examining the effect of administering 50-10% oxygen during first stage of labor actually had no effect on fetal scalp pH, and trended towards a worsening base deficit with supplemental O2. Another study of primates administered O2 with acidotic fetuses by scalp pH demonstrated worsening of acidosis with O2 administration.
These studies though, like the others, were small and nonrandomized. There is also criticism in the timing and application of O2 in each of these trials.
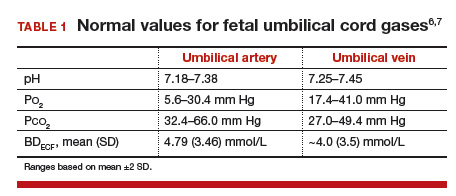
Non-inferiority RCT: a 2018 RCT in JAMA used a non-inferiority approach to randomize 114 patients to supplemental O2 versus room air with category II EFM. They found no difference between groups in improving umbilical artery lactate, which was their primary marker for this trial.
There was also no difference in other cord gas components or rates of cesarean delivery for fetal indications.
Umbilical artery lactate does have some ability to predict hypoxia-associated morbidity in neonates; however, it is not sensitive or specific for poor outcomes, a valid criticism. The trial was not powered for neonatal outcomes.
A secondary analysis of this same RCT looked at umbilical venous O2 concentration and actually found lower O2 pressure in fetuses exposed to long periods of O2 than those exposed for short periods or on room air.
The physiologic arguments for (or against) O2
Check out our fetal circulation episode for a quick review of how blood and oxygen travel in the fetus!
The maximum fetal PO2 (i.e., in the umbilical vein at the site of the placenta) cannot exceed maternal venous PO2. This is why fetal hemoglobin has to have a very high oxygen affinity, as it must extract O2 away from the venous side of maternal blood, which already is at a lower oxygen concentration.
An oxygen dissociation curve. Fetal hemoglobin maintains relatively excellent saturations, even at usual venous O2 pressures in maternal circulation (HbA). Source: WIKIPEDIA.
A normal venous Po2 in adults is around 35-45 mmHg (arterial is around 100 mmHg). That would equate on a HbA dissociation curve to a saturation of around 65-75%.
A normal Po2 in a venous cord gas, by comparison, is on average around 35mmHg, again representing maternal venous O2 tension.
But the fetal hemoglobin affinity for O2 powers this to about an 80-90% saturation! And that is considered normal -- most O2 saturation values at the 5 minute Apgar are in the mid-80%s.
The question lies herein: by causing maternal hyperoxemia, will that result in fetal recovery if the fetus is hypoxic?
By increasing the PaO2 in the mother with supplemental oxygen, theoretically there would be an increased oxygen gradient to diffuse downstream to the fetus.
In effect, because there is more oxygen tension, the higher the maternal PvO2 and umbilical vein O2 pressure can become.
But as we discussed with ischemic events, sometimes oxygen may counterintuitively not improve outcomes, or mask worsening of the process!
In this case, the fetus becomes hypoxic, or the “ischemic” tissue -- would the new O2 load in this case be detrimental?
Or potentially, like in COPD, would the normoxemia actually mask worsening acidosis?
Or finally, as demonstrated in the RCT we referenced, does the O2 even get to the fetus due to some placental transfer failure in the presence of hyperoxia?
What should the bottom line takeaway be?
That’s the other interesting thing about this -- in spite of the fact that there is little evidence supporting this practice, O2 is wildly popular as a resuscitative effort. It’s simple and quick to apply.
Intrauterine resuscitation, defined as repositioning, oxytocin discontinuation, fluid administration, amnioinfusion, or oxygen administration in response to fetal heart rate tracing abnormalities, are all options.
While we couldn’t identify any studies that shared the “natural history” of what’s done during a deceleration, anecdotally we know that reflexively, reaching for the facemask oftentimes will precede these other measures, despite the evidence on decelerations favoring these other options. In short, leave O2 for maternal hypoxia, or as a last-resort option for fetal resuscitation!