Espresso: Umbilical Cord Gas Interpretation
/Building somewhat on our fetal circulation episode from last week, today we’ll talk about umbilical cord gases. From an obstetrics perspective, these can be challenging to really interpret, but the simple interpretation is often worth some CREOG points if you can analyze these systematically.
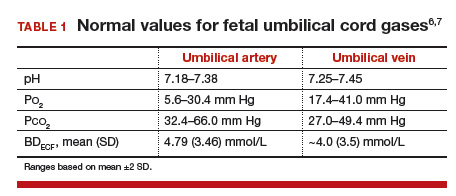
Remember, the umbilical vein is carrying oxygenated blood, and the umbilical arteries are carrying deoxygenated blood. This can help you remember the normal values, as they’ll be opposite those for an ABG versus VBG on an adult. Additionally, the umbilical vein is originating at the site of the placental interface with the mother -- so venous pHs will give a sense of maternal acid-base status, or the acid-base status at this interface. For this reason, the arterial pH is more helpful to truly measure fetal acid-base status.
The components of the blood gas are:
pH: represents the inverse log of the concentration of hydrogen ions in the circulating blood, or how acidic the blood is. In essence, more acid represents a lower pH, which represents more compromise.
Normal value for a venous pH is around 7.35 (as it is in adult blood).
Normal value for an arterial pH is around 7.28.
pO2: the pressure of oxygen (in essence its concentration) in fetal blood.
pCO2: similarly, the pressure/concentration of CO2 in fetal blood.
The pO2 and pCO2 can given additional clues to help with non-straightforward (i.e., mixed) acidosis.
Base Excess/Deficit: in blood, acid is buffered by bicarbonate ions. The base excess or deficit represents how much difference there is between those bicarbonate ions and hydrogen ions in order to return to a normal pH value of 7.35 in the umbilical vein. An excess is more bicarb; a deficit is less bicarb. However, these tend to get used interchangeably, and in these acid-base status questions, you’ll see the “excess” written as a negative number — implying what is actually a deficit.
Normal values for base excess are around 4 mmol/L in both the umbilical artery and vein.
A more significant base deficit signifies a metabolic acidosis -- i.e., the process causing insult has been longstanding, and there has been time to utilize bicarbonate to buffer the acid.
A lower base deficit signifies a respiratory acidosis -- i.e., the process has been acute, so there has been no buffering of the hydrogen ions.
A base deficit of 12 mmol/L has been suggested as severe, and more suggestive of metabolic acidosis.
(c) MDEdge
What about administering more O2 to the mother? Won’t that help things and reduce the fetal risk of acidosis?
If only it were that simple! Sadly, the answer is no. In most cases, maternal hemoglobin is fully saturated on room air. Fetal hemoglobin has a greater O2 avidity, and will pull O2 across the placental circulation. But when maternal blood is already saturated, the fetus won’t get any more O2 even if you pump it up to 4000L a minute by mega face mask! Some studies have suggested the additional free O2 in maternal serum may actually lead to vasospasm and cause harm!
The exception to this certainly is a change in maternal oxygenation or an indication for maternal O2 use -- but these indications suggest that maternal Hb is less than 100% saturated.
When should I get a cord gas?
It’s a good idea to practice the technique for cord gas collection, which requires collecting a 10-20cm doubly-clamped (i.e., proximally and distally) cord segment. Even on routine, vigorous deliveries, getting into this habit as part of your deliveries will help you be prepared.
Cord gases are not recommended to be sent with delayed cord clamping, so don’t send these if DCC is part of your practice! However, collecting the cord segment can be good practice for those learning proper technique.
There are no consensus rules about when to send a cord gas sample. At our institution, the general thought is “if you think you need one, send one.” However, common scenarios where cord gas sampling can be helpful to at least set aside on a “just in case basis” include:
Nonvigorous infant at delivery (i.e., Apgars at 5 mins less than 7)
Category III or “bad category II” tracings
Operative deliveries performed for NRFHT
Multiple gestation
Premature infants
Meconium stained fluid
Growth restriction
Breech deliveries
Shoulder dystocia
Intrapartum fevers or chorioamnionitis
Obviously this list is non-exhaustive, but goes to show there are a lot of indications! Some literature has suggested even universal arterial blood sampling at delivery may be cost-effective.
The best way to learn this is to do some practice cases. Check out the below resources for some practice questions and further explanations.